General Decontamination
CellDrop instruments can be decontaminated using standard cell culture laboratory chemicals (e.g., 70% EtOH or 10% bleach).
WARNING: Do not spray or pour cleaning solutions directly on the instrument. Instead, spray disinfectant solution on a laboratory wipe or paper towel and then wipe the instrument.
Do not use acetone or hydrofluoric acid on or around the instrument. These compounds will damage the instrument.
Normal Cleaning Between Samples
Best practice:
- Lift the arm.
- Fold a standard laboratory wipe. For best results do not crumple or wad up the wipe.
- Wipe the lower surface once with a single, firm stroke (Figure 1).
- Repeat for the upper surface.
- Do not wipe a surface back and forth multiple times. This could cause a static charge and a loading problem.
- Lower the arm and verify in the live preview that the sample surfaces are clean.

Loading Issues
A loading issue occurs when the sample does not enter the sample chamber or the sample is not loading evenly in the field of view. Loading issues can be caused by dried sample, accumulation of sticky media or from a static charge. Pipetting plain water or trypan blue dye without cells can also load the counting chamber poorly. It is recommended to test sample loading with a cell or bead sample. In the event of a sample loading problem, simple steps will restore the ability to normally load samples.
Ethanol Sample Surface Cleaning
Cleaning with 70% ethanol removes dried sample and media. In rare instances, residue accumulation can create sample loading issues. Ethanol cleaning is a best practice recommended at that start and conclusion of each cell counting session.
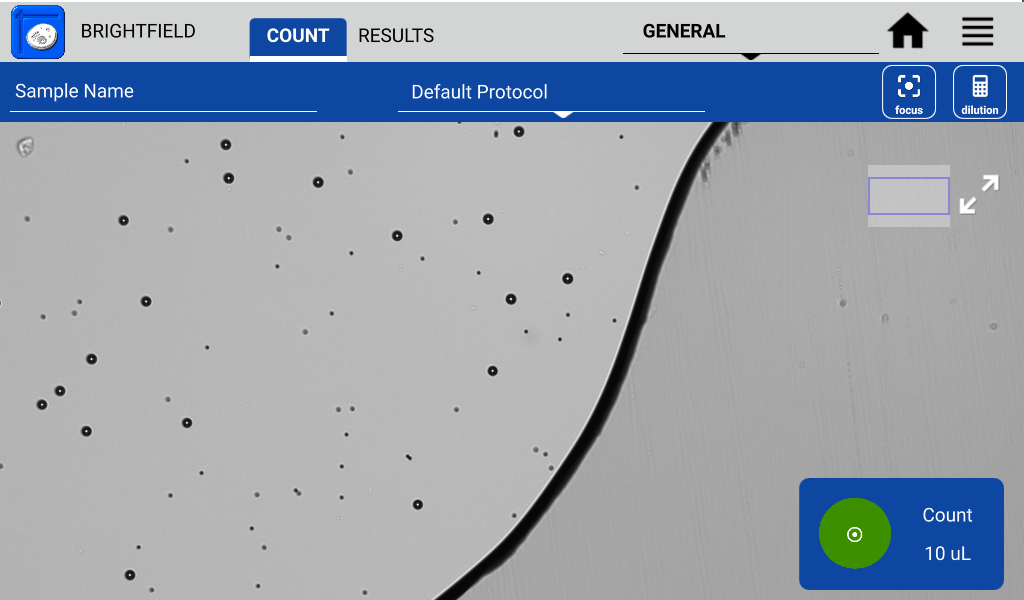
- Open any Count app.
- Lower the arm to start the live preview.
- Select the default protocol which sets the chamber height at 100 µm.
- Load 15 µL of 70% ethanol into a pipette.
- Place the pipette tip in the alignment groove (Figure 4) and then dispense.
- Allow the solution to remain in the sample chamber 15-20 seconds.
- With a folded lab wipe, wipe each surface in one direction using a single, firm stroke (Figure 5).
- Lower the arm and verify on screen that the sample surfaces are clean.
- Repeat if necessary to ensure that all cells and debris have been cleaned from the alignment groove and sample surfaces.
Note: If loading issues persist repeat steps 4 – 8 using 10% Bleach


Removing Static Charge
A static charge can be easily dissipated to restore normal loading.
- Spray 70% ethanol onto a large laboratory wipe.
- Wipe down the black metal surface on the top of the instrument and the lower sample surface (Figure 6).
- Wipe down the black metal parts of the arm and the upper sample surface (Figure 7).


Additional Resource – Loading and Cleaning Video
31-JUL-2024