Introduction
The accurate measurement of yeast cell density and viability is essential for brewers aiming to optimize fermentation performance, minimize batch variability, and maintain product consistency. While viable yeast samples play a pivotal role in the brewing process, assessing yeast cell viability only provides information on live and dead cells in a whole population. Vitality is a measurement of how metabolically active a yeast cell is and, therefore, is an indicator of the health of a living cell and the suitability of its environment to growth. Viability is a prerequisite for vitality, allowing researchers to differentiate metabolically active cells from a dormant living cell population.
Current yeast vitality methods, such as acidification power tests1 or the measurement of CO2 production with gas production systems2, are time-consuming, expensive, and impractical at the industry scale. Because of its difficulty, brewers will often instead count the number of pitches a yeast sample is used and replace it with a new yeast batch after 5 – 10 generations.3
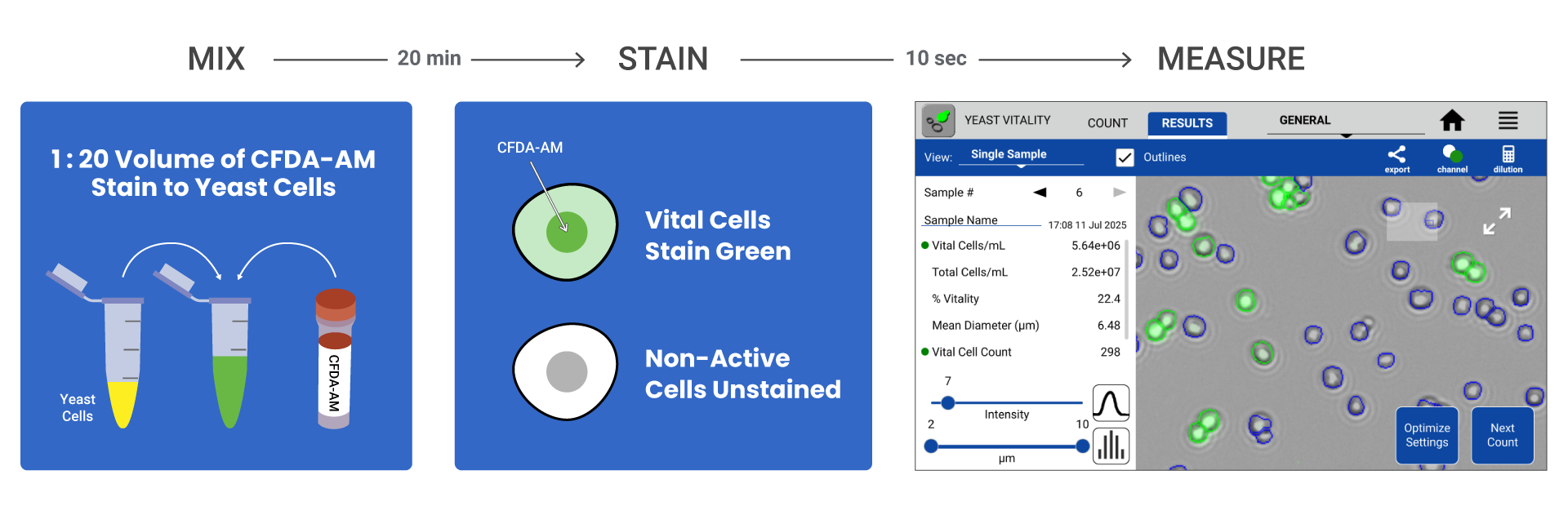
A simple and affordable alternative method for accurately measuring yeast vitality can be achieved by using 5-CFDA-AM (CFDA-AM) stain in conjunction with the Yeast Vitality App on the CellDrop FLxi Automated Cell Counter (Figure 1). The CFDA-AM molecules accumulate inside live active yeast cells. In metabolically active cells, the molecule is enzymatically activated by intracellular esterases. This process produces a green fluorescent stain in metabolically active cells while dead or dormant cells will remain non-fluorescent.
This technical note uses French Saison yeast assayed in three distinct physiological states (log phase, room temperature slurry, and slurry maintained at 4℃) to demonstrate the utility of the 5-CFDA-AM assay to determine metabolic activity in yeast.
Materials
- French Saison Yeast Slurry (White Labs Part No.: WLP590)
- 5-CFDA-AM (MedChemExpress, Cat. No.: HY-131131)
- DMSO (Millipore Sigma, Cat No.: D2653)
- Yeast Dilution Buffer (PBS)
Protocol
Five milligrams of the CFDA-AM reagent was first dissolved in 1 milliliter of DMSO and further diluted in DMSO to a working concentration of 380 μM.
Three distinct French Saison yeast culture conditions were prepared for vitality assessment: 1) Log Phase Culture—an actively growing overnight culture, 2) Room Temperature Slurry—yeast slurry equilibrated to room temperature, 3) Cold Storage Slurry—a volume of slurry sampled from 4℃ storage.
The overnight inoculation was prepared using fresh Yeast Extract Peptone Dextrose (YPD) growth media and French Saison yeast stock and allowed to incubate for 14 hours on a shaker at 30°C and 300 rpm. The overnight yeast sample was counted on the CellDrop FLxi and then diluted in growth media to a target cell density of 4 x 106 and incubated 30°C at 300 rpm for four hours to allow the sample to reach the log growth phase.
The cold storage slurry sample was maintained at 4℃, and the room temperature slurry was left at room temperature for four hours before vitality staining and measurement. All of the samples’ Yeast cells/mL densities were determined using a simple Brightfield total cell count on the CellDrop FLxi and diluted appropriately in PBS buffer to 1 x 106 cells/mL immediately prior to the staining procedure.
To stain the yeast cells, 5 µL of the 380 µM CFDA-AM working stock solution was combined with 95 µL of diluted yeast sample to a final ratio of 1:20. Cells were incubated away from direct light in microcentrifuge tubes at room temperature for 20 minutes. Following incubation, samples are loaded directly onto the CellDrop FLxi for analysis.
Results
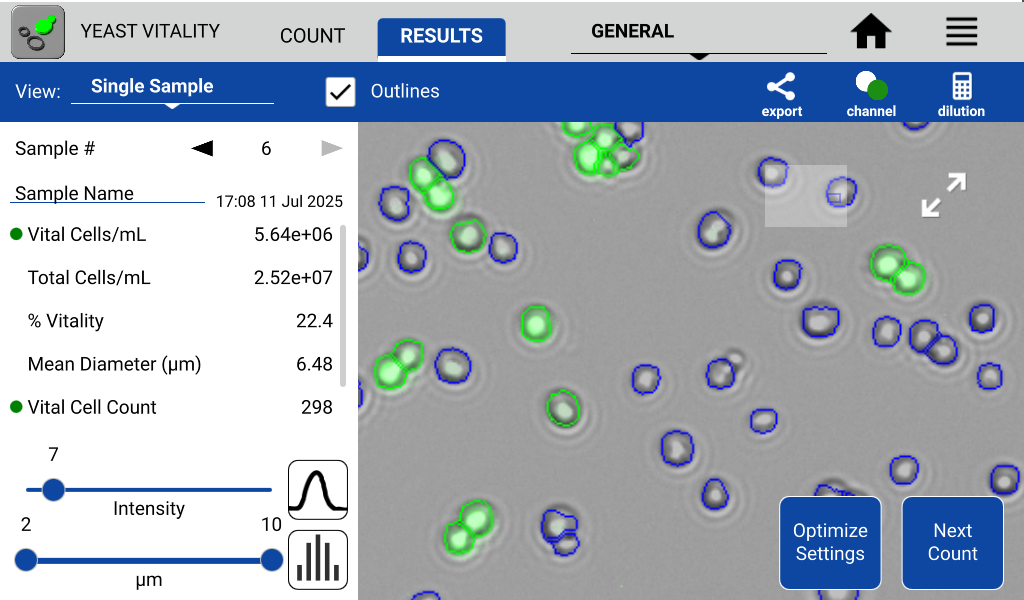
To test the utility of 5-CFDA-AM for assessing metabolic activity in yeast, three different conditions of the same French Saison culture were prepared and measured in triplicate on the CellDrop FLxi using the Yeast Vitality App. Post measurement the CellDrop FLxi’s Yeast Vitality App automatically calculates Vital cells/mL, Total cells/mL, and % Vitality of the yeast cell population. Representative results, including visual identification of vital (green outline) and non-vital (blue outline) cells, are shown in Figure 2.
The CellDrop FLxi also allows for easy development and recall of strain-specific measurement protocols, and all data is automatically saved onboard for convenient access and archiving.
As anticipated, yeast cells in the log phase growth condition exhibited the highest metabolic activity and thus the greatest vitality (99.8% vital). This contrasted sharply with a significant reduction in vitality observed for the room temperature slurry (38% vital) and cold storage slurry samples (30% vital), reflecting their diminished metabolic activity under less favorable conditions (Figure 3).
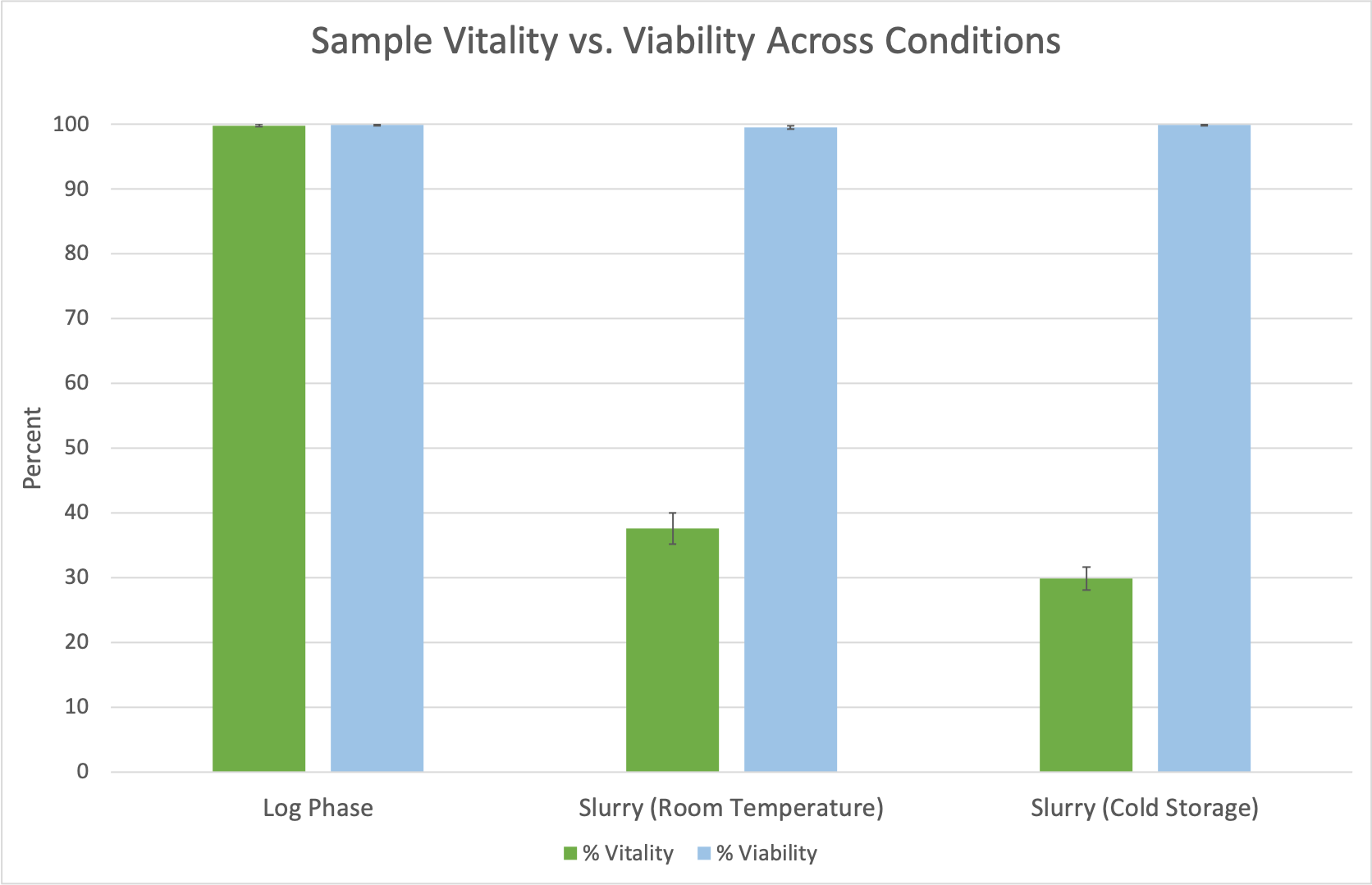
To confirm that differences in vitality were due to metabolic activity rather than cell death, yeast viability was also assessed for all conditions using the simple AO/PI Viability assay on the CellDrop FLxi. Viability across all three conditions remained greater than 99% across all three conditions (also shown in Figure 3). This data confirms that the observed reduction in vitality for the room temperature and cold storage slurries was attributed to decreased metabolic activity in live but dormant cells, rather than an increase in dead cells. This distinction underscores the importance of measuring vitality to gain a comprehensive understanding of yeast health beyond simple live / dead counts.
Summary
This technical note demonstrates that the CellDrop FLxi Automated Cell Counter, combined with the 5-CFDA-AM stain and its dedicated Yeast Vitality App, provides a rapid, affordable, and accurate method for assessing yeast vitality. Unlike traditional methods that are often time-consuming and costly, this automated solution offers results in seconds.
In brewing, understanding yeast vitality is crucial, as it directly reflects the metabolic activity of live yeast cells—a key factor influencing fermentation performance and beer quality. While viability (live vs. dead cells) is a prerequisite, vitality offers deeper insight into the health and readiness of the yeast population. By automating vitality measurements, brewers can quickly process more samples faster and at a lower cost with reduced user-to-user variability.
Ultimately, the CellDrop FLxi offers an efficient and reliable solution for yeast health monitoring, enabling brewers to optimize pitching rates, monitor fermentation rates, and reduce batch variability.
Click here to learn more about the CellDrop Automated Cell Counter or request a quote.
Click here to learn more about our other yeast cell counting solutions.
References
- Stewart, Graham G. Distilling and Brewing Yeast. Springer, 2017.
- Michel, Maximilian, et al. “Optimisation of Yeast Vitality Measurement to Better Predict Fermentation Performance.” Journal of the Institute of Brewing, vol. 126, no. 2, 27 Feb. 2020, https://doi.org/10.1002/jib.604.
- Yeast Harvesting & Repitching – Wyeast Lab. (2021, October 27). Wyeast Lab. http://wyeastlab.com/resource/professional-yeast-harvesting-repitching/
9-OCT-2025