Associated Kits
- KIT-CD-FL-DEMO [K-30600]
- KIT-CD-AO-PI-1.5 [K-30520]
- KIT-CD-AO-PI-7.5 [K-30530]
Kit Contents
Kits include a premixed solution of AO and PI in PBS. The reagents should be stored protected from light at 2 – 8oC in an airtight container.
| Assay Size | Number of Tests |
|---|---|
| 0.25 mL | 50 |
| 1.5 mL | 300 |
Sample Volume and Chamber Height
The required sample volume for the CellDrop depends on the height of the measurement chamber, which is set in the counting protocol.
Standard Magnification (FLi)
| Gap Height (um) | Volume (uL) | Minimum Density (cells/mL) | Maximum Density (cells/mL) |
|---|---|---|---|
| 400 | 40 | 7.0E+02 | 3.1E+06 |
| 100 | 10 | 2.9E+03 | 1.3E+07 |
| 50 | 5 | 5.9E+03 | 2.5E+07 |
| Gap Height (um) | Volume (uL) | Minimum Density (cells/mL) | Maximum Density (cells/mL) |
|---|---|---|---|
| 400 | 40 | 4.3E+03 | 2.6E+07 |
| 100 | 10 | 1.7E+04 | 1.0E+08 |
| 50 | 5 | 3.4E+04 | 2.1E+08 |
Best Practices
- Ensure that the upper and lower chamber surfaces are clean prior to loading sample.
- Lower the arm prior to dispensing sample into the measurement chamber.
- Mix the cell suspension well immediately prior to loading sample, and avoid introducing air bubbles.
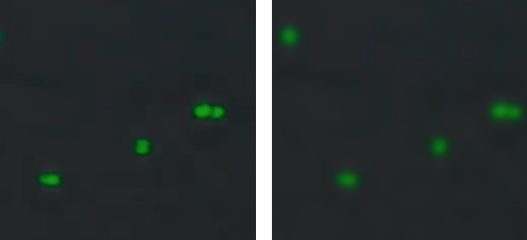
- Follow the image guides to adjust focus and fluorescence exposure.
- Allow cells to settle and stop moving across the live preview before pressing the Count button.
- Adjust exposure in the green and red channels so that fluorescent cells are not over or underexposed, as shown in the info dialog in the exposure menu.
Sample Prep
- Mix cell suspension well. Allow AO/PI to equilibrate to room temperature and vortex briefly.
- Mix AO/PI and cell suspension together in a 50% solution (1 part AO/PI + 1 part cell suspension = Dilution Factor of 2).
- Note: There is no incubation time required. Fluorescence may start to fade if cells are in AO/PI for more than 30 minutes.
- Mix sample thoroughly prior to loading onto the CellDrop.
Sample Measurement
- With the CellDrop arm in the down position, launch the AO/PI app.
- Set sample name, information and protocol as appropriate.
- Pipette well-mixed cells + AO/PI solution and dispense appropriate sample volume into the measurement chamber, using the groove on the lower sample surface as a pipetting guide.
- Note: The volume of sample required depends on the protocol settings for the chamber height. The required volume is displayed on the Count button.
- Adjust focus according to the image guide. Set initial focus in the brightfield channel, then refine focus in the green channel.
- Switch to green and red channels and adjust exposure according to the image guide.
- Allow cells to settle, then press the Count button.
Refer to Technical Note 186 – CellDrop Best Practices or Technical Note 197 – AO/PI Fluorescence Assay vs. Trypan Blue for additional guidance.
Refer to denovix.com/sds for safety data sheets for CellDrop Cell Counting Assays.
21-MAY-2025