Associated Kits
- KIT-CD-PI-APOP-0.1 [K-30610]
Kit Contents
The Apoptosis assay kit is available in 100 assay sizes and includes 100 µL of 50 ug/mL Annexin V FL Conjugate, 100 uL of 20 ug/mL Propidium Iodide (PI), and 10.2 mL of 5X Annexin V binding buffer. The kit should be stored at 4 degrees Celsius or kept on ice when in use. The Annexin V FL Conjugate and PI should be kept protected from light. Please note 0.01M PBS is required for this assay but not provided in the kit.
| Assay | Component 1 | Component 2 | Component 3 |
|---|---|---|---|
| DeNovix Apoptosis Assay | Annexin V FL Conjugate | Propidium Iodide | 5X Annexin V Binding Buffer |
| 100 samples | 100 µL | 100 µL | 10.2 mL |
Sample Volume and Chamber Height
The required sample volume for the CellDrop depends on the height of the measurement chamber, which is set in the counting protocol.
Higher Magnification (FLxi)
| Gap Height (um) | Volume (uL) | Minimum Density (cells/mL) | Maximum Density (cells/mL) |
|---|---|---|---|
| 400 | 40 | 4.3E+03 | 2.6E+07 |
| 100 | 10 | 1.7E+04 | 1.0E+08 |
| 50 | 5 | 3.4E+04 | 2.1E+08 |
Best Practices
- Ensure that the upper and lower chamber surfaces are clean prior to loading sample.
- Lower the arm prior to dispensing sample into the measurement chamber.
- Mix cells immediately before loading sample and avoid introducing air bubbles.
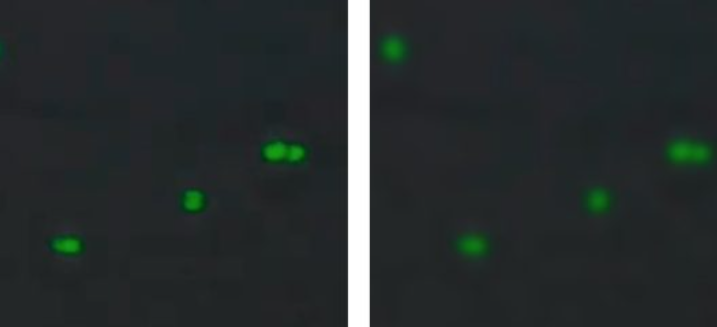
- Follow the image guides and focus offset tool (Figure 1) to adjust focus and exposure in brightfield (Figure 2), the green channel (Figure 3) and in the red channel (Figure 4).
- Allow cells to settle and stop moving across the live preview before pressing the Count button.



Sample Prep
- Treat Cells or induce apoptosis in cells using treatment of choice and appropriate positive control. Recommended cell concentration per replicate range: 1 x 10^6 to 5 x 10^7 per mL.
While apoptosis is being induced or cells are being treated, perform the following steps in an ice bucket or similar:
- Dilute 5X Annexin V binding buffer 1:5 using distilled water, prepare approximately 0.6 mL of 1X binding buffer prepared for each sample.
- Spin the tubes of 100 µL of 50 ug/mL Annexin V FL Conjugate and the 20 ug/mL PI for 15 seconds at 10,000 x g or at max speed using a benchtop mini centrifuge to ensure all the liquids are at the bottom of the tubes and place the tubes on ice.
- After treatment and incubation, harvest cells by centrifugation and wash with PBS.
- Discard supernatant and resuspend cells in 0.5 mL of 1X Annexin V binding buffer.
- Aliquot cells into 1.5 mL or smaller tubes at 8 µL per sample replicate when using the 100 micron chamber height.
- Add 1 µL of the 50 ug/mL Annexin V FL Conjugate and 1 µL of 20 ug/mL PI to the 8 µL per replicate. Or adjust volume according to the number of desired replicates.
- Samples for single stained controls should also be prepared by adding either 1 µL of Annexin V FL Conjugate or 1 µL of PI to respective tubes containing 8 µL of Annexin V binding buffer and samples mix.
- Incubate the samples at room temperature for 15 – 30 minutes in the dark.
- After which, 10 uL of 1X binding buffer should be added to each tube of a single replicate (or 10 * number of replicates per tube to get the correct volume of 1X Annexin V buffer added to each tube) and placed on ice to arrest the apoptotic process and proceed to sample measurement.
Sample Measurement
The stages of apoptosis can then be analyzed by detecting Annexin V FL Conjugate in the green channel and PI in the Red channel using the Apoptosis app on the CellDrop FLxi Automated Cell Counter.
- With the CellDrop arm in the down position, launch the Apoptosis app.
- Set sample name, information, and protocol as appropriate.
- Load the unstained and the one stain controls and gate each channel appropriately.
- Set the focus, intensities, and exposures properly.
- Adjust focus according to the image guide. See Best practices above.
- Switch to green and red channels and adjust exposure according to the image guide.
- Pipette well-mixed cells + Apoptosis solution and dispense appropriate sample volume into the measurement chamber, using the groove on the lower sample surface as a pipetting guide.
- Note: The volume of sample required depends on the protocol settings for the chamber height. The required volume is displayed on the Count button.
- Allow cells to settle, then press the Count button.
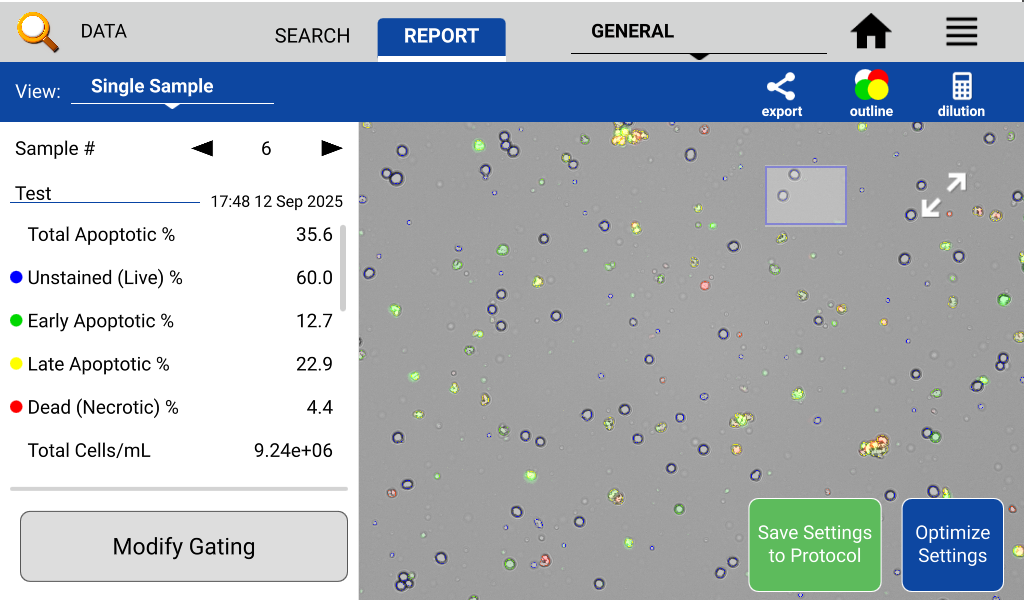
- If needed, use the modify gating button to check and adjust the fluorescent and size gating settings for Live, Dead or Apoptotic cells.
Refer to Technical Note 186 – CellDrop Best Practices for additional guidance.
Refer to denovix.com/sds for safety data sheets for CellDrop Cell Counting Assays.
21-OCT-2025