Materials and Methods
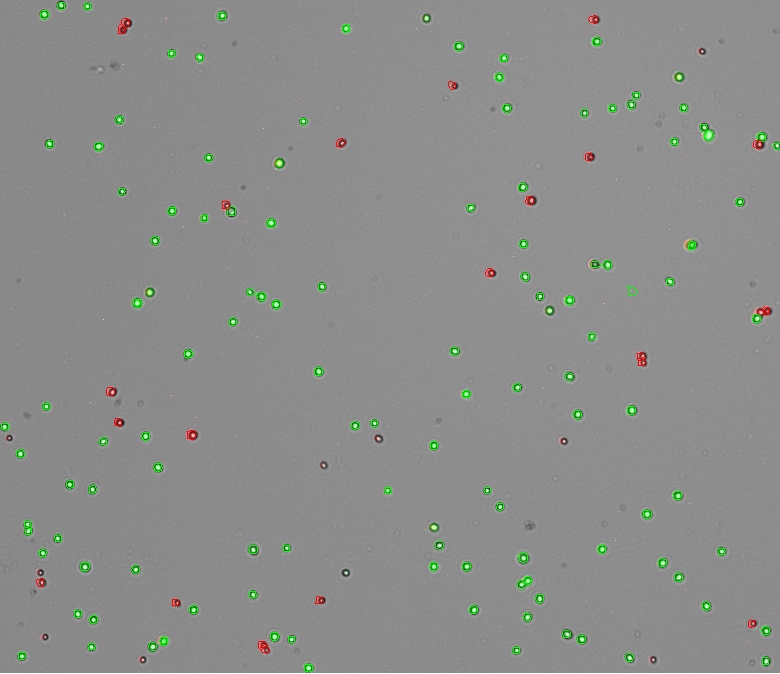
Measurements were performed using the DeNovix Yeast Assay kit. Fresh S. cerevisiae sample cultured at room temperature in YPD media was diluted to approximately 2×106 cells/mL with assay buffer. For each measurement, 18μL of yeast sample was combined with 1μL of FDA and 1μL of PI solutions. The reaction solution was mixed well and incubated at room temperature in the dark for 15 minutes. The sample was mixed by pipetting up and down before dispensing 10 μL into the CellDrop counting chamber.
Viability analysis was performed with the CellDrop FL in the Yeast app, which uses green and red filters to count live cells fluorescing green with FDA and dead cells fluorescing red with PI. The CellDrop Yeast App default protocol was used for the count.
Note: Protocol optimization may be required for different yeast strains
Protocol Settings
CellDrop EasyApps™ are designed to count a variety of cell lines with minimal customization. If necessary, the user can optimize count settings after a count has been taken or develop a new protocol. Protocols include several powerful settings to enable proper counting of all yeast cells in a sample while ignoring any debris that could compromise the count data. See Tech Note 189 – Count Settings for additional details.
Optimizing Count Settings
Count settings may be edited after a count is performed by pressing ‘Optimize Settings’ on the results screen. This feature allows a user to assess how a parameter change affects cell count versus the previous parameter/count. For more concentrated yeast samples, a chamber height of 50 µm is recommended to count higher densities and lower the settling time before counting the number of cells. Though some adjustments may still be required, the recommended settings for counting S. cerevisiae sample shown in Figure 1 are as follows:
| Count Application | Yeast |
| Chamber Height | 100 µm |
| Dilution Factor | 1.11 |
| Diameter(min) | 2 µm |
| Diameter (max) | 10 µm |
| Live Roundness | 1 |
| Dead Roundness | 1 |
| Green Fluorescence Threshold | 1 |
| Red Fluorescence Threshold | 1 |
Summary
Automated counting of yeast samples on the CellDrop FL removes operator variability from the process and speeds up the workflow. The Yeast app reports data for total, live, and dead cell counts in terms of counted cells and cells/mL, as well as mean cell diameter and percent viability. The CellDrop FL with dual-color fluorescence has powerful counting algorithms and customizable count settings to enable accurate counting and viability determination of a wide variety of cell types.
Further information:
04-APR-2023